Q(D1). What is a passive sampler?
A. The terminology originated for sampling analytes in air. In an active sampling device air is pumped to the analyte removal site, whereas for passive sampling it simply diffuses there. The same terminology was adopted for water samplers that rely on diffusion.
Q(D2). What is a passive water sampler?
A. A device that can be submerged in situ in a water body where it accumulates the analyte automatically by it diffusing to a binding site. Analysis of the accumulated analyte is performed later after retrieval.
Q(D3). What does DGT mean?
A. It stands for Diffusive Gradients in Thin-Films, which describes the mechanism of DGT where a controlled diffusion gradient is established in a layer of gel. It can be used as a simple passive sampler and as a more versatile tool for probing chemical and biological processes.
Q(D4). What is a dynamic technique?
A. From a physical chemistry perspective DGT is a dynamic rather than an equilibrium technique because it continuously removes analyte from the medium. In this respect DGT is like voltammetry, but simpler. Information can be gained on speciation in solution, and solid phase to solution transfer in soils and sediments because of the dynamic response of the medium to the dynamic removal.
Q(D5). What is the basic principle of passive water sampling?
A. Removal of analyte to a binding layer establishes a concentration gradient within the passive sampler. From the amount bound measured in the laboratory Fick’s law of diffusion can be used to calculate the insitu concentration.
Product Advice
Q(A1). What is the meaning of the product codes?
A. The product code provides a fairly full description of the product. It is based on the composition of the device rather than the analyte, as some devices can be used for several, often diverse, analytes.
Take a typical example: LSNM-NP
L signifies loaded, meaning that the plastic housing comes complete with the gel and membrane layers, so that it is ready for deployment.
SN signifies that it uses the plastic housing that is appropriate for deployments in solution. Alternatives are SL (the housing appropriate for deployments in soils) and SP, indicating a sediment probe.
M signifies that it uses a binding layer of Chelex100. The complete list of codes for binding layers is given below. In most cases the choice of letters is not intuitive.
B Spheron thiol
C XAD resin
D HLB
E NMDG
H AgI
M Chelex100 resin
P Ferrihydrite
R Activated charcoal
T Metsorb (TiO2)
V TEVA resin
X Mixture of Chelex and Metsorb
Y Mixture of Chelex and Ferrihydrite
Z Zirconium oxide
N signifies the type of diffusion layer used. N corresponds to the normal APA polyacrylamide gel cross-linked with an agarose derivative. The alternative is A, which corresponds to an agarose gel.
P signifies the type of membrane filter used. P indicates polyethersulphone. Alternatives are G, indicating GHP, N, indicating Nuclepore and T, indicating PTFE.
Q(A2). Which DGT is best for measuring cations and oxyanions together?
A. Generally we would recommend a device with a mixed binding layer of Metsorb (TiO2) and Chelex. This is the most robust combination which generally performs well in both freshwater and seawater.
For deployments in all types of waters you will need device LSNX-NP
For deployment in soilsA you will need device LSLX-NP
For deployments in sedimentsB you will need LSPX-NP
Details of the performance of this binding layer can be found in:
- G. Panther, W. W. Bennett, D. T. Welsh and P. R. Teasdale, Simultaneous measurement of trace metal and oxyanion concentrations in water using diffusive gradients in thin films with a chelex-metsorb mixed binding layer, Anal. Chem., 86: (2014), 427-434.
Although a binding layer that uses ferrihydrite instead of Metsorb has some performance limitations, especially for deployments longer than a few days in seawater, it does have advantages in some situations. Devices with a mixed Chelex and ferrihydrite binding layer are preferred where analysis is by total digestion of the binding layer, elution is restricted to a single acid step, or for phosphate where the final analysis is colorimetric.
For deployments in all types of waters you will need device LSNY-NP
For deployment in soilsA you will need device LSLY-NP
For deployments in sedimentsB you will need LSPY-NP
Q(A3). Is the type of diffusive gel layer important?
A. It is important for several reasons. First it must be reasonably thick to ensure that the rate of water flow has a negligible effect on the measurement. Second it must be sufficiently strong to ensure a robust device that can be readily manufactured and deployed in demanding conditions. Thirdly it must not interact with the analyte, as this can affect the measurement. Research has shown that the diffusive gel we use in most products, a polyacrylamide gel crosslinked with an agarose derivative, known as APA, best meets these requirements. Charged species, such as trace metal cations and anions, interact with gels that themselves have a charge. When correctly prepared, there is negligible charge on the APA gel, even at ionic strengths of 1 mM, and so DGT measurements can be interpreted with confidence. This is not the case if agarose is used as the binding layer. This gel has a distinct negative charge at ionic strengths less than 10 mM, and consequently DGT measurements in freshwaters of di or trivalent cations, using this as the diffusion layer, are overestimated, while anions are underestimated. Some of our competitors supply such devices without pointing out these limitations. We only use agarose as the diffusive layer for analytes with little charge where it will not affect the measurement, particularly for organic compounds and uni-valent species.
Practicalities
Q(P1). How long should I deploy DGT?
A. Deployments in water
In principle DGT can be deployed for a wide range of times from a few hours to a few months and interpretation of the concentration using the standard simple DGT equation will be valid. However, several factors can limit this range. These are the concentrations of the analytes, the selectivity and capacity of the analyte and binding layer combination, the presence of competitive ions, the extent of complexation in solution, the possibility of analyte adsorption to the diffusive layer and the possibility of biofilm formation.
For simple cationic trace metals and a Chelex binding layer, deployment times between 3 days and 3 weeks should be optimal. If the concentrations of the metals are low (less than a few micrograms per litre) and there is no indication of biofilm growth on the surface of the devices, longer times may be appropriate. The deterrent to using shorter times is that complexation in solution may reduce the time taken to reach steady state accumulation. In waters with low concentrations of organic matter or very high metal concentrations, this effect will be minimal and so deployment times as short as a day can be used.
Similar deployment times can be used when measuring oxyanions, including phosphate using a Metsorb binding agent, but times in excess of two weeks would not be recommended for deployments in waters of high ionic strength such as seawater.
Data for the most appropriate deployment times for the measurement of mercury are sparse. Conservatively it would be sensible to deploy between 3 days and 2 weeks.
Early indications are that times of between 3 days and 3 weeks should be suitable for organic compounds.
Deployments in soils and sediments
After a maximum reached within a few hours, the flux to a DGT device deployed in a soil or sediment will progressively decline as the analyte adjacent to the device is consumed. If the requirement is to calculate concentration, a deployment of 1 day is appropriate. Longer deployment times up to three days may, however, be appropriate in some circumstances. For example, if trace metals are strongly complexed by humic substances, the slower diffusion of these complexes delays the time to maximum flux. Deployment of 3 days will overcome this slower approach to a pseudo steady state. When probes are inserted into soils and sediments there will inevitably be a small temporary disturbance of the spatial distribution of analyte. This spatial structure is rapidly restored through the strong redox buffering mechanisms. However, If there is a desire to measure metals at high spatial resolution in sediments or soils, leaving the probes in place for longer than 1 day will allow better establishment of the structure and minimise the effect of the initial disturbance on the time-averaged measured flux.
Q(P2). Where do I find the diffusion coefficients I need?
A. The most comprehensive listing of diffusion coefficients of mainly inorganic substances appropriate for DGT measurements can be found in W. Davison and H. Zhang, Diffusion Layer Properties, Chapter 3, in: Diffusive Gradients in Thin-films (DGT) for Environmental Measurements, Editor: William Davison, Cambridge University Press, 2016. A list of consensus values for metal ions at various temperatures is available (Diffusion Coefficients). Simple algorithms are also provided (adapted from the Appendix of the above DGT book) to enable calculation of values at any temperature from 1 to 35oC.
There are no clear consensus values for mercury, as can be seen from the tables of Chapter 3 in the DGT book. However, Suggested values of diffusion coefficients for mercury deduced from available knowledge and information are available.
As new methods for measuring organics using DGT are developed, diffusion coefficients are being determined, as provided in Information for organic analytes. While theses values are expected to be reliable, it is advisable to recognise that generally there are as yet insufficient data to establish them as consensus values.
Q(P3). Which equation should I use to calculate concentration in solution?
A. In most situations where DGT is deployed in water that is flowing or subject to convection currents the standard DGT equation is appropriate.
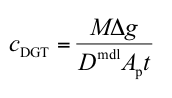
cDGT (nmol or ng mL-1) is the concentration of analyte in the deployment medium measured by DGT.
M (nmol or ng) is the mass of analyte accumulated in the binding layer.
Δg (also known as δg) (cm) is the total thickness of the materials that comprise the diffusion layer (gel and filter membrane).
Dmdl (cm2 s-1) is the diffusion coefficient of analyte in the material diffusion layer.
Ap (cm2) is the physical area of the exposed filter membrane.
t (s) is the deployment time.
Δg and Ap are known properties for the supplied DGT device. The time of deployment, t, is known by the operator. DMDL is also available provided the deployment temperature is known. The accumulated mass, M, is measured in the laboratory. Usually it is obtained by eluting the analyte and calculating the mass from the measured concentration in the known volume of eluate. Recommended units to facilitate easy calculation are shown.
This standard DGT equation incorporates an automatic correction for a modest diffusive boundary layer (DBL) at the surface of the device. Such a modest DBL thickness will apply when there is good solution flow over the surface of the DGT device. In this case cDGT is likely to be accurate to ±5%. More generally when DGT devices are deployed in natural waters with unknown hrdrodynamics, because of uncertainties in the thickness of the DBL due to the local hydrodynamics and deployment configuration, cDGT should be regarded as accurate to ±20%.
If greater accuracy is required the DBL thickness should be measured in situ using 3 or more DGT devices with different values of Δg. Then a fuller equation is required. Detailed accounts of the most appropriate calculation procedure for different circumstances are available in W. Davison and H. Zhang, Principles of measurements in simple solutions, Chapter 2, in: Diffusive Gradients in Thin-films (DGT) for Environmental Measurements, Editor: William Davison, Cambridge University Press, 2016 and in W. Davison and H. Zhang, Progress in understanding the use of diffusive gradients in thin-films – back to basics, Environ. Chem. 9: (2012), 1-13. These articles also consider when the necessary requirement that the time taken to reach a steady state transfer of analyte to the DGT device is negligible compared to the deployment time.
The most commonly used equation for multiple devices is
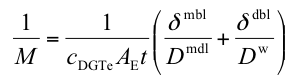
AE is the effective area which has a value of 3.8 cm2 for standard solution devices. δmdl is the combined thickness of diffusive gel and filter membrane and δdbl is the thickness of the diffusive boundary layer. Concentration, in this case denoted by cDGTe, and δdbl can be obtained from linear plots of 1/M versus δmdl.
The above treatments assume that there are no competition effects or limitations to the capacity of the binding layer, which will generally be true. Calculations can still be made if these situations apply, using the approaches and equations provided in M. Jimenez-Piedrahita, A. Altier, J. Cecilia et al., Extending the use of diffusive gradients in thin films (DGT) to solutions where competition, saturation, and kinetic effects are not negligible, Anal. Chem, 89: (2017), 6567-6574.
Q(P4). Can I calculate concentration from deployments in soils or sediment?
A. Yes you can. The basic DGT equation can be used.
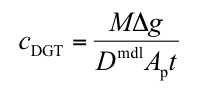
cDGT (nmol or ng mL-1) is the mean concentration of analyte measurable by DGT in the pore water adjacent to the surface of the DGT device, averaged over the total deployment time.
M (nmol or ng) is the mass of analyte accumulated in the binding layer.
Δg (also known as δmbl) (cm) is the total thickness of the materials that comprise the diffusion layer (gel and filter membrane).
Dmdl (cm2 s-1) is the diffusion coefficient of analyte in the material diffusion layer.
Ap (cm2) is the physical area of the exposed filter membrane or the area of sliced gel that is measured.
t (s) is the deployment time.
The key thing here is that because DGT continually removes analyte, its concentration at the surface of the DGT device may be lowered during the course of the deployment. In some soils and sediment the analyte may be continually resupplied to solution from the solid phase. When this effective buffering is substantial, the measured cDGT is the same as the concentration that would be measured directly on the porewaters. Comparison of cDGT with alternative measurements of concentrations in porewaters (or soil solution) can provide information on the dynamics of analyte exchange between porewater and solid phase.
A detailed appreciation with thorough referencing of the principles involved in deploying DGT in soils and sediments can be found in
- J, Lehto, Principles and applications in soils and sediments, Chapter 7, in: Diffusive Gradients in Thin-films (DGT) for Environmental Measurements, Editor: William Davison, Cambridge University Press, 2016
Q(P5). How should I store DGT devices and for how long?
A. According to our quality tests, most binding gels (except precipitated ferrihydrite gel) and diffusive gels (except bis-acrylamide cross-linked polyacrylamide gel known as BPA) can be stored at room temperature for more than one year in a well-sealed container with 0.01-0.03 M NaNO3 or NaCl solution.
For loaded DGT devices packed in a plastic bag, evaporative leakage could induce dehydration of diffusive gels and affect DGT performance. To eliminate this impact, we normally add 0.4 mL of NaNO3 or NaCl solution to each plastic bag for long term storage. There was no significant influence on measurements using DGT devices stored in these bags at room temperature for one month and at 4oC for six months. The small amount of solution remaining in the plastic bag should not present a problem. The DGT devices can be deployed directly on removal from the bag.
Q(P6). Should I treat and analyze my sample directly after deployments?
A. The concentrations and speciation of analytes should not change after they bind to the binding gels. Thus the DGT devices can be stored in well controlled conditions until it is convenient to analyse them.
We strongly recommend rinsing your devices with MQ/deionized water immediately after retrieval. This treatment is important to get rid of any solid particles or microorganisms attached to the device’s surface, as there is the potential for these solid phases to leak further analyte during transportation or storage.
If the storage time prior to analysis is longer than two weeks dehydration may be a problem. It can make the separation of binding and diffusive gels difficult. We recommend that, for times longer than 2 weeks, DGT devices are packed in individual clean plastic bags with a small volume of MQ/deionized water.
Q(P7). What should I do if the binding gel cannot be separated from the diffusive gel?
A. In most situations, this problem can be solved by adding 100 – 150 μL of MQ/deionized water to the gel surface and waiting for 10 – 20 mins. If the problem still exists, we suggest that the diffusive and binding gels are eluted together. Vgel (mL) here is close to the volume of water used for rehydration provided that all water is absorbed by the gels. M (nmol or ng) is the mass of analyte accumulated in the binding layer. X is the dilution time. fe is the elution factor. Ve (mL) is the volume of eluent. Cicp (nmol/mL or ng/mL) is the concentration measured by ICP-MS or other relevant instruments.
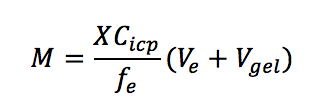
Q(P8). Should I deoxygenate sediment probes?
A. The simple answer is ideally yes, but there are other considerations. Because the DGT assembly is thin it does not contain a lot of oxygen and, within a short period of time, (say 30 minutes) the oxygen within the DGT gels will be consumed by the oxygen diffusing out into the sediment and electron donors diffusing into the gels. Clearly this will compromise the DGT measurement of redox sensitive and secondary affected components (e. g. trace metals) within this 30 minute time period of initial accumulation. However, DGT is usually deployed for times in excess of a day. Therefore, this initial period where the presence of oxygen can have an effect on the DGT accumulation will only represent about 2% of the total accumulation time. Consequently the effect on the DGT measurement is likely to be negligible. There is an urgent need for work to be done to verify experimentally the effect of preliminary deoxygenation on DGT measurements.
Even deoxygenation of the DGT probes is not so straightforward because it will only be effective if the transfer of the DGT probes from their deoxygenation solution to the sediment can be done within no more than a few 10s of seconds. Exposure of the probe to the air for longer times will quickly replenish the oxygen, as demonstrated in Davison, W., Zhang, H. & Grime, G.W. (1994). Performance characteristics of gel probes used for measuring the chemistry of pore waters. Envi. Sci. Technol. 28, 1623-1632.
Q(P9). Is the DIFS dynamic model available?
A. When a DGT passive sampler is deployed in a soil or sediment it continuously removes the analyte of interest from the immediately adjacent porewater. If there is a dynamic equilibrium between solute in the solid phase and in solution, the locally lowered concentration in the porewater causes resupply of solute from the solid phase to solution. Harper et al1,2, developed a numerical model of the dynamics of DGT in contact with a porous solid phase medium, known as DGT induced fluxes in sediments or soils, DIFS. Key terms within DIFS are the size of the pool of labile analyte in the solid phase and the rate of release of the analyte from solid phase to solution. Ernstberger et al3, showed that by measuring the mass accumulated by DGT when it is deploying in homogenized soils for different times it was possible to use DIFS to obtain the labile pool size and the dissociation rate. In other experiments where there are alternative estimates of the pool size, DIFS can be used to calculate release rates directly4,5. DIFS was initially formulated as a one-dimensional model, perpendicular to the DGT surface, but later a two-dimensional version became available6. A thorough review of the development and application of DIFS, its limitations and other modelling possibilities is available in the DGT book7.
To obtain the software, please email Hao Zhang directly: h.zhang@lancaster.ac.uk
- P. Harper, W. Davison, H. Zhang, W. Tych, Kinetics of metal exchange between solids and solutions in sediments and soils interpreted from DGT measured fluxes. Geochimica et Cosmochimica Acta,, 62:16 (1998), 2757–2770.
- P. Harper, W. Davison, W. Tych, DIFS—a modelling and simulation tool for DGT induced trace metal remobilisation in sediments and soils. Environmental Modelling & Software, 15:1 (2000), 55-66.
- Ernstberger, W. Davison, H. Zhang, A. Tye, S. Young, Measurement and dynamic modeling of trace metal mobilization in soils using DGT and DIFS. Environmental Science and Technology, 36:3 (2002), 349-354.
- Zhang, E. Lombi, E. Smolders, S. McGrath, Kinetics of Zn Release in Soils and Prediction of Zn Concentration in Plants Using Diffusive Gradients in Thin Films. Environmental Science and Technology, 38:13 (2004), 3608-3613.
- J. Fitz, W. W. Wenzel, H. Zhang, J. Nurmi, K. Stipek, Z. Fischerova, P. Schweiger, G. Kollensperger, L. Q. Ma, G. Stingeder, Rhizosphere characteristics of the arsenic hyperaccumulator Pteris vittata L. and monitoring of phytoremoval efficiency. Environmental Science and Technology, 37:21 (2003), 5008-5014.
- Ł. Sochaczewski, W. Tych, B. Davison, H. Zhang, 2D DGT induced fluxes in sediments and soils (2D DIFS). Environmental Modelling & Software, 22:1 (2007), 14-23.
- J. Lehto, Principles and Applications in Soils and Sediments, In: Diffusive Gradients in Thin-Films for Environmental Measurements, Ed. W. Davison, Cambridge University Press, Cambridge, 2016, pp146-173.
Q(P10). How can I check the performance of DGT?
A. The simplest way is to deploy DGT in a well stirred solution of known analyte concentration for a known time (for trace metals, normally using 10 ppb Cd in 10 mM NaNO3 solution and deploying DGT devices for 4 hours). DGT continuously removes analyte from solution, so it is necessary to have sufficient volume of solution to ensure that the concentration of analyte isn’t depleted during the deployment. If one standard DGT device is deployed in 1 L of solution for 1 day it will reduce the concentration of the analyte in that solution by about 1.5%, a negligible error. In practise several devices may be used in the same solution. For example, they may be removed at different times to obtain a plot of mass versus deployment time, which should be linear. In this case a solution volume of a few litres may be appropriate and deployment times might be 4, 8, 12, 16, 20 and 24 h.
The following protocol is adapted from Chapter 10 in the DGT book.
Quality control: blanks and performance tests
Each laboratory that uses DGT should ideally verify its performance. This not only establishes that the DGT devices are performing well, but also verifies that the handling and analytical procedures used in the laboratory are appropriate. The simplest way to do this is to deploy DGT in a known solution in the laboratory. It is essential that all containers and solutions are scrupulously clean. The procedure is described for Cd, which is not prone to contamination and does not adsorb to the diffusive layer or membrane filter. A mixed metal solution prepared from neutral salts could be used or a single metal solution of choice. As Cu and Pb can adsorb to the diffusion layer materials, the deployment time at their concentration of 10 µg L-1 needs to be 24 h to ensure accuracy. An alternative supporting electrolyte to NaNO3 could also be used, including synthetic lake water or seawater.
Prepare a deployment solution of 10 µg L-1 of Cd in 10 mM NaNO3 by diluting 20 mL of 1M NaNO3 solution to 2 L in a 3 L plastic container. Add an appropriate small aliquot of Cd standard solution (prepared from neutral salt to ensure the solution is not acidic). Check the pH is between 5 and 7. Place a large magnetic follower in the deployment solution and put the container onto a magnetic stirrer. Place the DGT devices into a suitable holder that can be placed against the container wall, while maintaining the plane of the front filter membrane vertical. A simple plastic assembly will suffice, such as those available from DGT Research. Immerse in the solution, which should then be stirred well. Use an appropriate stirrer which does not heat up during use and whose speed is well regulated. Simple stirrers can be used, but, if available, it is best to use one that can maintain a constant stir rate that can be displayed. A good guide is that the solution should not cavitate but should be on the verge of doing so. Take a small solution sample for analysis and note the time and temperature. After approximately 4 h, sample the solution again, measure the temperature and then retrieve the DGT devices, noting the time of retrieval. Rinse the devices with high purity water and then dismantle, separating the cap from its base by twisting a screwdriver inserted in the grove in the base. Place the Chelex gel in a clean sample tube, such as a 1.5 ml microcentrifuge vial, and add 1 ml of 1 M HNO3, leaving for 24 h or at least overnight. The Cd concentration in this eluent should be about 20 µg L-1 assuming the test temperature was about 25oC. For analysis by ICP-MS a 10x dilution of the eluent is recommended.
The DGT blank is obtained using a Chelex gel disc after it has been retrieved from a DGT device which was not deployed.
Calculation
For this simple test which uses a standard DGT device, with 0.8 mm thick diffusive gel and a membrane filter, deployed in a well stirred solution, a simple calculation procedure can be used. Chapter 2 in the DGT book provides various calculation options, depending on the device parameters and deployment conditions and Chapter 1 provides the basic equations that can be used here. The accumulated mass, M, is calculated from the measured concentration of metal, ce in the 1 M HNO3 eluent, taking account of the volume of the binding layer, Vbl, 0.16 ml in this case and the volume of the eluent solution, Ve, 1 ml in this case (equation 1).
1. 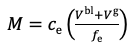
The elution factor, fe, compensates for the fact that only a proportion of metal is released on elution. For Al, Ca, Cd, Co, Cu, Fe, Mg, Mn, Ni, Pb, Zn, U, REEs metals, a universal value of 0.85 can be used. For Cr(III), 0.8 is preferable. When using equation 1 remember to express concentration in units of mass per mL, if the volumes are in mL. The concentration of metal measured by DGT, cDGT, can be calculated using equation 2.
2. 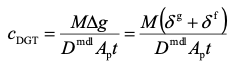
The combined thickness of the diffusive gel (dg = 0.08 cm) and filter (df = 0.013 cm) is Δg, D is the diffusion coefficient of metal through this material diffusion layer and Ap is the physical area of the exposure window (3.14 cm2). Tables of diffusion coefficients at different temperatures are available on the web site. Diffusion coefficients in a standard Supor filter membrane are the same as in the standard APA diffusive gel. The units of cDGT will be in mass per cm3 if Δg is in cm, t in s and D in cm2 s-1.
Target performance
The measured value of cDGT should be within 10% of the solution concentration measured directly in the samples of deployment solution collected during the experiment. If there is a greater difference, first check the calculation and then rerun the experiment, being especially aware of contamination issues. Blanks values will be useful in assessing possible contamination. Acceptable values for the mass (ng) measured on the blank discs are: Cd and Co, 0.02; Mn, Pb and U, 0.1; Cu, Ni and P, 1; Zn, 10.
DGT Book: Diffusive Gradients in Thin-Films for Environmental Measurements, Ed. W. Davison, Cambridge University Press, 2016
